
Immune Cell Therapy Lab (Lab - 6)
Centre for Stem Cell Research,
Christian Medical College Campus,
Bagayam, Vellore - 632002.
Office: +91 416 228-5134
Lab: +91 416 228-5127
Fax: +91 416 228-5103
Career Interests
- CAR-T cell Therapy for Haematological Malignancies
- Genome editing of T-cells
- Virus Specific T-cells
Education
- Post-Doctoral Research Fellow (2017-2023): St. Jude Children’s Research Hospital, Memphis, TN, USA.
- PhD in Biochemistry and Biomedical sciences (2012-2007): Department of Haematology, Christian Medical College, Vellore, India.
- M. Phil in Endocrinology (2007-2008): PGIBMS, University of Madras, Chennai, India.
- MSc in Biochemistry (2005-2007):Thiruvalluvar University, Vellore, India.
Awards
- DBT-Ramalingaswami re-entry fellowship (2023).
- Abstract Achievement Award Recipient, American Society of Hematology Annual Meeting (2013, 2015 and 2020), USA.
- Young scientist travel award from Department of Science and Technology, India (2015).
- Young scientist travel award from Department of Biotechnology, India (2013).
- Indian council for Medical Research (ICMR) Senior Research Fellowship (2014-2017).
- Silver medal in M.Sc. (Thiruvalluvar University).
Research
Generation of off-the-shelf allogenic anti-CD19 CAR-T cells for B-cell malignancies
In recent years, autologous chimeric antigen receptor (CAR) T-cell therapy has revolutionized the treatment landscape for haematological malignancies. Following the remarkable clinical outcomes, the Food and Drug Administration (FDA) approved six CAR-T cell products for B-cell leukemia, non-Hodgkin lymphoma and multiple myeloma. However, the safety, efficacy, and accessibility of this therapy have been hampered by exhaustion and poor persistence of infused CAR-T cells in vivo, cytokine-related toxicities, antigen escape by tumor cells, and bottlenecks in the production of autologous products. Alternatively, using allogeneic CAR-T cells from healthy donors has several potential benefits over autologous approaches, such as the immediate availability of cryopreserved batches for patient treatment, the ability to modify cells over time, redosing or combination of CAR-T cells targeting different targets and reduced cost using an industrialized process. However, allogeneic CAR-T cells may cause fatal graft-versus-host disease (GvHD) and may be rapidly rejected by the host immune system.
Therefore, we aim to generate off-the-shelf allogenic anti-CD19 CAR-T cells by two different strategies (described below) to avoid the host immune rejection and GvHD. In the first approach, we plan to generate the CD19 CAR-T cells from healthy donors followed by gene editing to eliminate T-cell receptor alpha constant (TRAC) and Beta-2 microglobulin (2M) genes to avoid graft-versus-host-disease (GVHD). In the second approach, we plan to develop combination immunotherapy, in which healthy donors T-cells will be primed with cytomegalovirus (CMV) followed by lentiviral transduction of anti-CD19 CAR to eliminate CD19+ tumor cells and the risk of inducing GVHD in the allogeneic hematopoietic cell transplant (HCT) setting, by removing alloreactive T-cells. The anti-CD19 CAR-T cells developed by both modalities will be tested for their tumor-killing potency in vitro and in vivo.
Strategy-I: Generation of allogenic CD19 CAR-T cells using base editors:
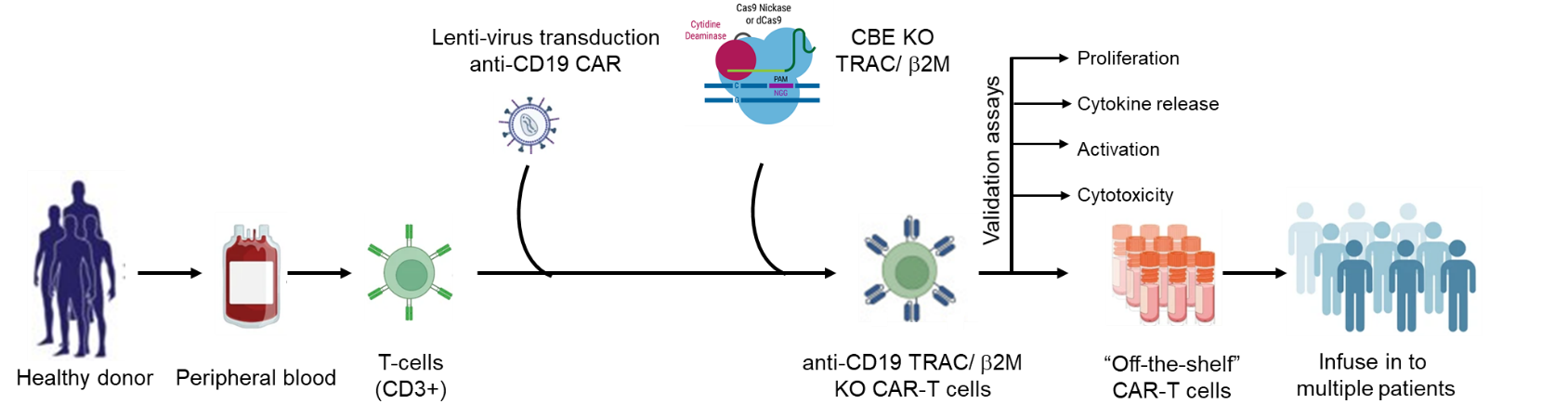
T-cells (CD3+ cells) will be purified from the peripheral blood of healthy volunteers by magnetic-activated cell sorting (MACS) separation. Selected T-cells will be transduced with lentivirus carrying CD19 CAR followed by electroporation of C-base editor (CBE) mRNA and sgRNAs to introduce the stop codon in T-cell receptor alpha constant (TRAC) and Beta-2 microglobulin (b2M) genes. CD19 CAR-T with TRAC and b2M knockdown cells will be further stimulated and expanded in vitro to assess the potency of generated allogenic CAR-T cells by measuring the proliferation (standard cell count), activation (measuring CD25 and CD69 by flow cytometry), cytokine profile (measuring IL-2, TNFa and INFg by ELISA) and cytotoxicity activity by co-culturing with CD19+ malignant cells (NALM-6 and RAJI cell lines).
Strategy-II: Generation of virus primed bi-specific (CD19/20) CAR-T cells:
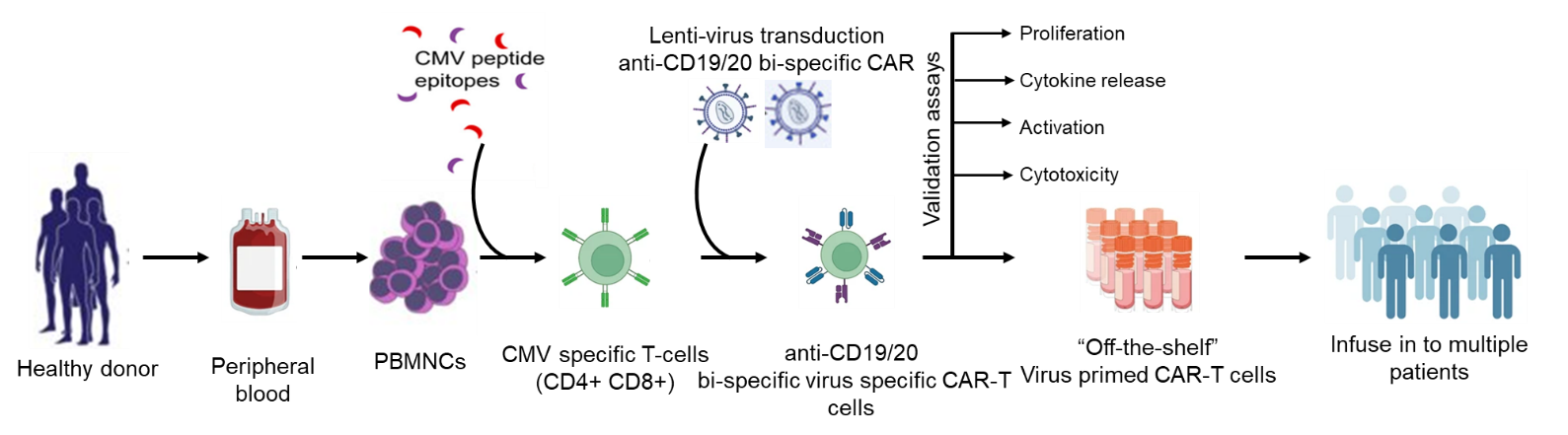
Peripheral blood mononuclear cells (PBMNCs) will be purified from the blood of healthy volunteers with certain serostatus. Then, PBMNCs will be cultured with CMV-specific peptides to generate virus-specific cells. Further, CMV-primed T-cells (CD4/8) will be selected and purified by MACS and flow cytometry based on CD4/8, IFNg and CD154 expression. Selected virus-specific T-cells will be transduced with lentivirus carrying CD19/20 bispecific CAR. Virus-primed CD19/20 bispecific CAR-T cells will be further stimulated and expanded in vitro to assess the potency of generated Virus primed allogenic bispecific CAR-T cells by measuring the proliferation (standard cell count), activation (measuring CD25 and CD69 by flow cytometry), cytokine profile (measuring IL-2, TNFa and INFg by ELISA) and cytotoxicity activity by co-culturing with CD19+ malignant cells (NALM-6 and RAJI cell lines).

Johnseena N M
Junior Research Fellow
E-mail: johnseena.nm@cmcvellore.ac.in
Research Interests: Generation of off-the-shelf CAR-T cells by gene editing
Education: M.Sc Genomic Science, Central University of Kerala, India.
Selected Publications
- Yoonjeong Jang, Ruopeng Feng, Lance E Palmer, Thiyagaraj Mayuranathan, Yu Yao, Kalin Mayberry, Sheng Zhou, Jian Xu, Jeffrey Michael Gossett, Guolian Kang, Yong Cheng, Jonathan S Yen, Mitchell J Weiss. BCL11A-deficient human erythropoiesis is impaired in vitro and after xenotransplantation into mice. Blood Advances. 2025 Feb 28:2024015574. 10.1182/bloodadvances.2024015574. [PMID 40020162].
- Senthil Velan Bhoopalan, Thiyagaraj Mayuranathan, Nana Liu, Kalin Mayberry, Yu Yao, Jingjing Zhang, Jean-Yves Métais, Koon-Kiu Yan, Robert E. Throm, Steven R Ellis, Yan Ju, Lei Han1, Shruthi Suryaprakash, Lance E. Palmer, Sheng Zhou, Jiyang Yu, Yong Cheng, Jonathan S. Yen, Stephen Gottschalk, Mitchell J. Weiss. Preclinical development of lentiviral vector gene therapy for Diamond-Blackfan anemia syndrome. Molecular Therapy. 2024 Dec 12:S1525-0016(24)00819-0. doi: 10.1016/j.ymthe.2024.12.020. [PMID 39673126].
- Thiyagaraj Mayuranathan, Gregory A. Newby, Ruopeng Feng, Yu Yao, Kalin D. Mayberry, Cicera R. Lazzarotto, Yichao Li, Rachel M. Levine, Nikitha Nimmagadda, Erin A. D. Dempsey, Guolian Kang, Shaina N. Porter, Phillip A. Doerfler, Jingjing Zhang, Yoonjeong Jang, Jingjing Chen, Henry W. Bell, Merlin Crossley, Senthil Velan Bhoopalan, Akshay Sharma, John F. Tisdale, Shondra M. Pruett-Miller, Yong Cheng, Shengdar Q. Tsai, David R. Liu, Mitchell J. Weiss, Jonathan S. Yen. Potent and uniform fetal hemoglobin induction via base editing. Nature Genetics. 2023 Jul;55(7):1210-1220. doi: 10.1038/s41588-023-01434-7 [PMID 37400614].
- Christophe Lechauve, Julia Keith, Alfonso G Fernandez, Eugene Khandros, Kalin Mayberry, Thiyagaraj Mayuranathan, Lance E Palmer, Xiaohui Qiu, Heather Sheppard, Rahul Telange, Hans-Martin Herz, Mitchell J Weiss. Ancestral β-globin gene haplotypes modify β-thalassemia severity in a mouse model. Blood Advances. 2024 Mar 27:bloodadvances.2024012681. doi: 10.1182/bloodadvances. [PMID 38536944]
- Kelcee A. Everette , Gregory A. Newby, Rachel M. Levine, Kalin Mayberry, Yoonjeong Jang, Thiyagaraj Mayuranathan, Nikitha Nimmagadda, Erin Dempsey, Yichao Li, Senthil Velan Bhoopalan, Xiong Liu, Jessie R. Davis, Andrew T. Nelson, Peter J. Chen, Alexander A. Sousa1, Yong Cheng, John F. Tisdale, Mitchell J. Weiss , Jonathan S. Yen 4 & David R. Liu. Ex vivo prime editing of patient haematopoietic stem cells rescues sickle-cell disease phenotypes after engraftment in mice. Nature Biomedical Engineering. 2023 Apr 17. doi: 10.1038/s41551-023-01026-0. [PMID 37069266].
- Florence Borot, Olivier Humbert, Gregory A Newby, Emily Fields, Sajeev Kohli, Stefan Radtke, George S Laszlo, Thiyagaraj Mayuranathan, Abdullah Mahmood Ali, Mitchell J Weiss, Jonathan S Yen, Roland B Walter, David R Liu, Siddhartha Mukherjee, Hans-Peter Kiem. Multiplex Base Editing to Protect from CD33-Directed Therapy: Implications for Immune and Gene Therapy. bioRxiv. 2023 Feb 23;2023.02.23.529353. [PMID: 36865281].
- Ruopeng Feng, Thiyagaraj Mayuranathan, Peng Huang, Phillip A Doerfler, Yichao Li, Yu Yao, Jingjing Zhang, Lance E Palmer, Kalin Mayberry, Georgios E Christakopoulos, Peng Xu, Chunliang Li, Yong Cheng, Gerd A Blobel, M Celeste Simon, Mitchell J Weiss. Activation of γ-globin expression by hypoxia-inducible factor 1α. Nature. 2022 Oct;610(7933):783-790. [PMID: 36224385].
- Senthil Velan Bhoopalan, Jonathan S Yen, Thiyagaraj Mayuranathan, Kalin D Mayberry, Yu Yao, Maria Angeles Lillo Osuna, Yoonjeong Jang, Janaka Ss Liyange, Lionel Blanc, Steven R Ellis, Marcin W Wlodarski, Mitchell J Weiss. An RPS19-edited model for Diamond-Blackfan anemia reveals TP53-dependent impairment of hematopoietic stem cell activity. JCI insight. 2022 Nov 22;e161810. [PMID 36413407].
- Kunhua Qin , Peng Huang, Ruopeng Feng, Cheryl A Keller , Scott A Peslak, Eugene Khandros, Megan S Saari, Xianjiang Lan, Thiyagaraj Mayuranathan, Phillip A Doerfler, Osheiza Abdulmalik , Belinda Giardine, Stella T Chou , Junwei Shi, Ross C Hardison, Mitchell J Weiss , Gerd A Blobel. Dual function NFI factors control fetal hemoglobin silencing in adult erythroid cells. Nature Genetics. 2022
Jun;54(6):874-884. [PMID: 35618846]. - Gregory Newby*, Jonathan Yen*, Kaitly Woodard*, Thiyagaraj Mayuranathan*, Cicera Lazzarotto, Yichao Li, Heather Tillman, Shaina Porter, Yu Yao, Kalin Mayberry, Kelcee Everette, Elizabeth Thaman, Christope Lechauve, Akshay Sharma, Jordana Henderson, Michelle Richter, Kevin Zhao, Shannon Miller, Tina Wang, Luke Koblan, Anton McCaffrey, John Tisdale, Theodosia Kalfa, Shondra Pruett-Miller, Shengdar Tsai, Mitchell Weiss, David R Liu. Base editing rescues sickle cell disease in human hematopoietic stem cells and in mice. Nature. 2021 Jul;595 (7866):295-302. [PMID 34079130]. (* Equal Contribution).
- Xianjiang Lan, Ren Ren, Ruopeng Feng, Lana C Ly, Yemin Lan, Zhe Zhang, Nicholas Aboreden, Kunhua Qin, John R Horton, Jeremy D Grevet, Thiyagaraj Mayuranathan, Osheiza Abdulmalik, Cheryl A Keller, Belinda Giardine, Ross C Hardison, Merlin Crossley, Mitchell J Weiss, Xiaodong Cheng, Junwei Shi, Gerd A Blobel. ZNF410 Uniquely Activates the NuRD Component CHD4 to Silence Fetal Hemoglobin Expression. Molecular Cell. 2020 Dec 1; S1097-2765(20)30784-X. [PMID 33301730].
- Jean-Yves Metais*, Phillip A Doerfler*, Thiyagaraj Mayuranathan*, Daniel E Bauer, Stephanie C Fowler, Matthew M Hsieh, Varun Katta, Sagar Keriwala, Cicera R Lazzarotto, Kevin Luk, Michael D Neel, S Scott Perry, Samuel T Peters, Shaina N Porter, Byoung Y Ryu, Akshay Sharma, Devlin Shea, John F Tisdale, Naoya Uchida, Scot A Wolfe, Kaitly J Woodard, Yuxuan Wu, Yu Yao, Jing Zeng, Shondra Pruett-Miller, Shengdar Q Tsai, Mitchell J Weiss. Genome editing of HBG1 and HBG2 to induce fetal hemoglobin. Blood advances. 2019 Nov 12;3(21):3379-3392. [PMID 31698466]. (*Equal Contribution).
- Syed Mohammed Musheer Aalam, Kannan Vrindavan Manian, Sumitha Prameela Bharathan, Thiyagaraj Mayuranathan, Shaji Ramachandran Velayudhan. Identification of Stable OCT4+ NANOG− State in Somatic Cell Reprogramming. Cellular Reprogramming. 2016 Nov;18(6):367-368. [PMID 27622636].
- Thiyagaraj Mayuranathan, Janakiram Rayabaram, Reena Das, Neeraj Arora, Eunice S Edison, Mammen Chandy, Alok Srivastava, Shaji R Velayudhan. Identification of rare and novel deletions that cause (δβ) 0‐thalassaemia and hereditary persistence of foetal haemoglobin in Indian population. European Journal of Haematology. 2014 Jun;92(6):514-20. [24471888].
- Thiyagaraj Mayuranathan, Janakiram Rayabaram, Eunice Sindhuvi Edison, Alok Srivastava, Shaji R Velayudhan. A novel deletion of β-globin promoter causing high HbA2 in Indian population. Haematologica. 2012 Sep;97(9):1445-7. [PMID: 22581004].
- Sukyee Kwok, Susan R Rittling, Nicola C Partridge, Chellakkan S Benson, Mayuranathan Thiyagaraj, Narasimhan Srinivasan, Nagarajan Selvamurugan. Transforming growth factor‐beta1 regulation of ATF‐3 and identification of ATF‐3 target genes in breast cancer cells. Journal of Cellular Biochemistry. 2009 Oct 1;108(2):408- 14. [PMID: 19582787].
Patents
- Systems and methods for base editing of HBG1/2 gene promoter and fetal hemoglobin induction. (SJ 20-0020, Status-pending).
CSCR/CMC
- Dr Alok Srivastava CSCR/CMC
- Dr Mohankumar KM, CSCR
Will be updated soon
Will be updated soon
