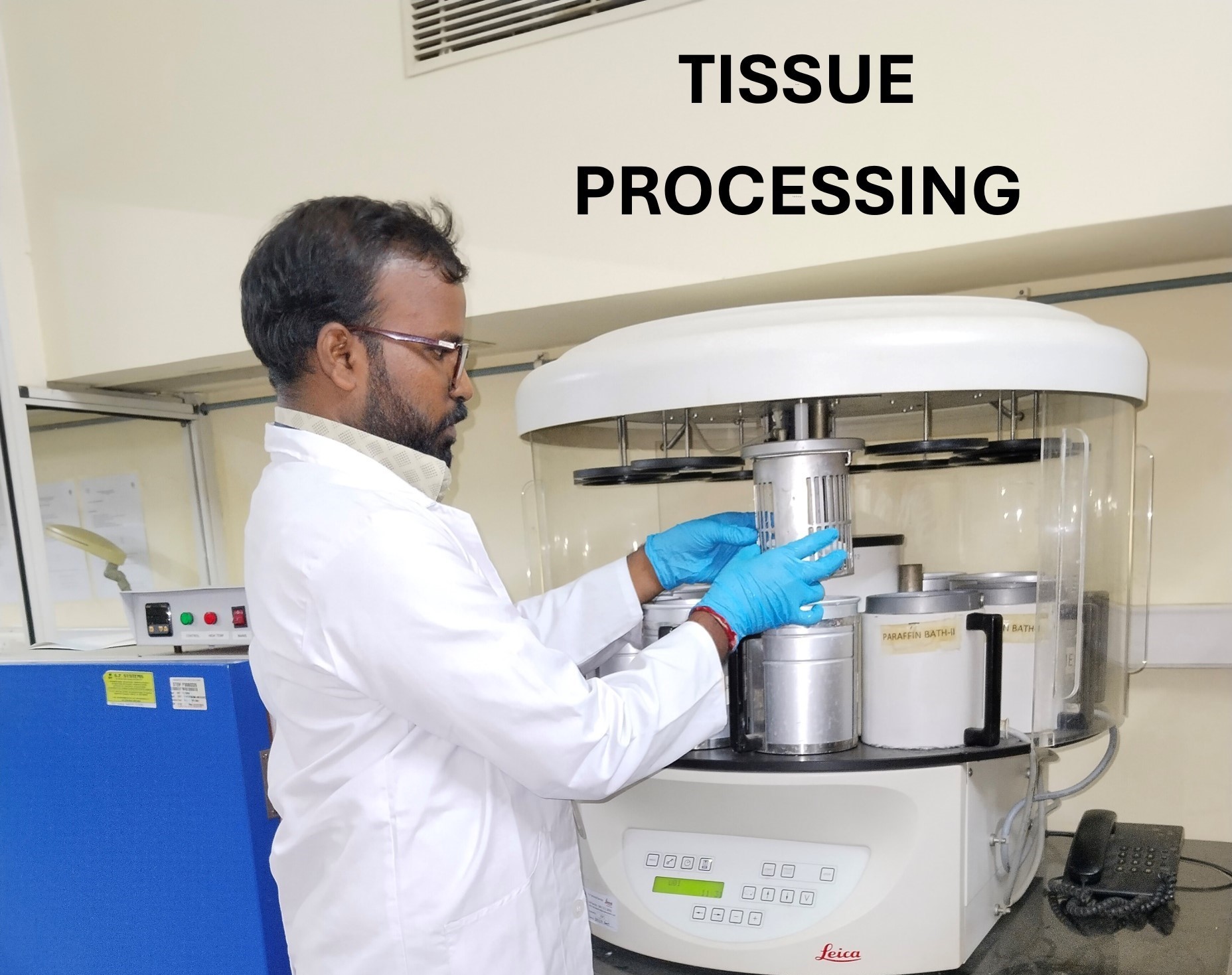
The Core Facilities at CSCR host state-of-the art instrumentation to aid researchers both within and outside CSCR.
The Core Facilities provide expertise in sample processing and analysis and also help in experiment design. All facilities are accessible to not only scientists working full time at CSCR but also to all other scientists in CMC, Vellore who require these technologies / platforms for their work.
Flow cytometry
Description
The CSCR Flow Cytometry and Cell Sorting Facility provide a broad array of instrumentation, support, education, and consultation to the research community. A wide variety of cell sorting modes are supported, from one-way to four-way tube cell sorting, Plate sorting, Slide sorting using high speed to low speed, different sizes of nozzles with 11 colors, and 13 parameters. Additionally, a wide variety of cell analysis services (up to 19 colors, 21 parameters) are offered. Currently, the facility offers one cell sorter (BD FACS Aria III) and two analyzers (BD FACS Celesta and BC Cytoflex LX) and two computers dedicated to the offline analysis of the flow cytometry data using FlowJo and Kaluza software.
Infrastructure
Facility In-Charge: Dr. Sandya Rani - Fellow-E (Scientist-D)
Technical Officer : Mr. A. Rajesh
Technical Staff : Mr. T. Abdul Muthallib, Mr. Joseph Joel N
Faculty Support : Dr. Sanjay Kumar
I. FACS Aria III:

The BD FACSAria III sorter is a superior multicolor performance, and legendary ease-of-use that opened the complex world of cell sorting to a broader audience of researchers and wider range of applications. A patented flow cell with gel-coupled cuvette and patented octagon and trigon detection system allow the system to achieve unrivaled sensitivity and resolution. BD FACSAria III cell sorter with a 5 laser 11 colour setup. The BD FACSAria III system has a throughput of 70,000 events per second and can do 4-way sorting and single cell sorting. Wavelength choices include 561-nm (Y/G), as well as the 488-nm (Blue), 633-nm (Red), 405-nm (Violet), and 375-nm (UV) lasers.
II. FACS Celesta:

BD FACS celesta is a multi-laser flow cytometer with 3 laser and 12 colour setup for delivering high sensitivity and performance. All configurations have blue (488-nm) and violet (405-nm) lasers, which has been paired with a yellow-green (561-nm) laser, for your application needs.
III. BC CytoFLEX-LX:

The High performance BC CytoFLEX-LX Flow cytometer analyser with Five High Power Lasers 488nm, 638nm, 405nm, 561nm and 355nm, 19 Colors & 21 Parameters (R3 B3-V5-YG5–UV3 System) is used for qualitative and quantitative measurement of biological and physical properties of cells and other particles. The system offers the ability to configure the violet laser detector (VSSC) to collect side scatter to better resolve nanoparticles from noise. It has Superior Acquisition rate of 30,000 events per second and Superior Signal Processing digital system with 7 decades dynamic range.
Imaging
Description
The CSCR Microscopy Core facility offers training and access to a variety of light and fluorescence microscopes; the core can also do imaging for users who are not trained and offer fluorescence and confocal imaging. The CSCR Microscopy Core is a full-service facility serving the research community. Our aim is to provide personalized assistance on all aspects of imaging, from tips on sample preparation to training on our microscopes to processing and analysis of image data. Our facility currently houses one Multiphoton Laser Scanning Microscope (OLYMPUS FV1000 MPE), confocal (OLYMPUS FV1000) and two fluorescence (EVOS FLAuto, LEICA DMI6000B), and four widefield light microscopes, and one computer dedicated to image processing and analysis.
Infrastructure
Facility In-Charge: Dr. Sandya Rani - Fellow-E (Scientist-D)
Technical Officer : Mr. A. Rajesh
Faculty Support : Dr. Saravanabhavan Thangavel
I. Inverted Fluorescence Microscope

The Leica DMI6000B is an inverted fluorescence microscope comprising of 6 interchangeable filters for detecting various fluorochromes. It has two independent cameras – DFC295 for high resolution bright-field imaging and DFC360 FX for high frame rate fluorescence imaging. It is also equipped with a fluorescence intensity manager and programmable function keys for easy access to functions.
II. EVOS FL Auto Imaging System

The EVOS® FL Auto Imaging System is a fully-automated, digital, inverted multi-channel fluorescence and transmitted light imaging system with outstanding workflow efficiency. Designed to meet demanding requirements over a broad range of applications, the EVOS® FL Auto system supports high-resolution mosaic tiling, multi-position well scanning, cell counting with thresholding, and time-lapse studies. The intuitive interface, proprietary light cubes, dual cameras, precision automated stage and parfocal optical system enables us to produce publication quality images in seconds. The EVOS® FL Auto system can be programmed to run acquisition routines, 8-point time lapse experiments, and tile-stitch scans in nearly any vessel through the sensitive touch-screen display.
III. Light Microscopes

Olympus BX43F upright microscope, Leica DMIL (upright) and Leica DMI1000 (inverted) microscopes are available for users to perform routine light microscopy imaging. DMIL and DMI1000 microscopes are provided with an interchangeable Leica DFC290 camera for high resolution bright-field imaging. The Leica DMI1000 is also installed in the tissue culture facilities of individual labs and the Core tissue culture area.
IV. Laser scanning confocal microscope system (Olympus FV1000)

The Olympus FV1000 confocal system comprises a motorized microscope with z focus drift compensation facility for bright field, differential interference contrast and fluorescence imaging with motorized XY scanning stage and CO2 incubation facility for live cell imaging. It is equipped with the following lasers - 405nm, Multi-Argon (458nm, 488nm and 515nm), 559nm and 635nm. Apart from regular confocal imaging, this microscope can be used to perform Multi-Area Time Lapse, FRET, FRAP, FLIM and diffusion experiments.
V. Laser scanning multi photon microscope (Olympus FV1000MPE).

The FV1000MPE is an upright multiphoton laser-scanning microscope coupled with a Mai Tai HP-Deep See–OL laser with automated broadband wavelength tuning from 690 to 1040nm for deep tissue imaging.
The systems supports multicolor fluorescent studies for imaging of living, whole mount or thickly sliced specimen. Dynamic biological processes can be imaged hundreds of micrometers within living cells and tissues. Provides support for applications where phototoxicity/photobleaching are a concern such as time course studies of living cells and tissues. Low magnification lens and long working distance stage allow imaging of large samples, embryos, and animals.
VI. Training Sessions
The Imaging Core Facility conducts training sessions regularly for both first time and experienced users. The training sessions comprise of specifically designed modules which include theory and practical sessions. The final authorization is given to the user upon successfully completing the required modules. The hands-on training sessions are tailored to the specific application requirement of each user so that they get the maximum benefit out of these systems. Apart from in-house training, the imaging core organizes sessions by application specialists from Leica and Olympus.
Molecular Biology
Description
The Molecular Core Facility is actively involved in providing the high end molecular biology services for the users (in house and off campus). The facility currently has a 3130 4-capillary DNA sequencer (Applied Biosystems), Quant Studio 12K Flex and 6K Flex Real-time PCR machines (Applied Biosystems) for high throughput analysis.
Many departments from CMC,Vellore and outside use this core facility extensively. The molecular biology core also aims to collaborate with people outside CSCR to share expertise and knowledge on platform development and augmentation.
Infrastructure
Technical Officer : Mr. A. Rajesh
Technical Staff: Ms. J. Saranya
Faculty Support : Dr. R. V. Shaji
I. Genetic Analyzer:

Genetic Analyzer 3130 is a 4 capillary series system with Electroosmotic flow suppression polymers (EOF). This system gives you all the advance automation with hands free operation and superior performance. This system provides compatibility with the existing application software systems, long-term reliability, automated polymer delivery system, enhanced thermal control, and optimized for multiple application.
II. Real-Time PCRs:

QuantStudio 12 K Flex system is designed for maximum throughput, outstanding flexibility with 5-interchangeable blocks, scalability and user friendly. This system is widely used in gene expression analysis, SNP genotyping, copynumber analysis, digital PCR technology, Micro RNA and other noncoding RNA analysis.
QuantStudio 6 K Flex system facilitates high-throughput analysis with 2-interchangeable blocks. This system is commonly used for PCR applications such as gene expression, gene regulation, gene variation and protein expression.
Radioactivity Core Facility

The Radioactivity Core Facility provides researchers a secure access to radiolabelled isotopes and instrumentation for detecting radioactivity. The facility currently has Greiger counters, GE Storm 365 Phosphor imager and a Perkin Elmer Tricarb Liquid Scintillation Counter.
Histopathology Laboratory
Histopathology Lab at the Centre for Stem Cell Research (CSCR) provides comprehensive histological services, including routine and special staining, as well as the newly introduced immunohistochemistry (IHC) and immunofluorescence (IF) services tailored for research and academic needs.
Infrastructure
Technical Staff: Mrs. Esther Rani, Mr. Ashok Kumar and Mrs. Farzana Imran
Faculty Support : Dr. Srujan Kumar Marepally
Specialized Staining Services:
We offer a range of special stains to meet diverse research requirements:
1.Histology Special Stains:
- Alcian Blue
- Acid Fast Bacillus stain for Mycobacterium Tuberculosis
- Elastic Van Gieson
- Hematoxylin and Eosin (H&E)
- Gordon Sweet Reticulin
- Masson Trichrome
- Masson Fontana
- Perl’s Prussian Blue Iron Stain
- Periodic Acid Schiff (PAS)
- Safranin-O
- Sirus Red Stain
- Toluidine Blue
- Verhoeff’s Elastic Stain
2.Cytology:
- Cytospin Preparation
- Cell Block Preparation
- Cryostat sectioning.
Immunohistochemistry (IHC) and Immunofluorescence (IF)
We are excited to announce that the Histology Lab at the Centre for Stem Cell Research (CSCR) now offers immunohistochemistry (IHC)/ immunofluorescence (IF) services, in addition to the existing routine and special staining options, for research and academic purposes.
- Standardized Protocol: Researchers must provide a standardized protocol for antigen retrieval
- Primary Antibody: You are required to supply the specific primary antibody along with your samples.
Cost Structure:
- Sample Embedding (Paraffin Block): Rs 245/sample
- PLL Slide: Rs 40/slide
- IHC Processing Charges: Rs 150/slide
For further details or inquiries, please contact us at: cscrhisto@cmcvellore.ac.in.
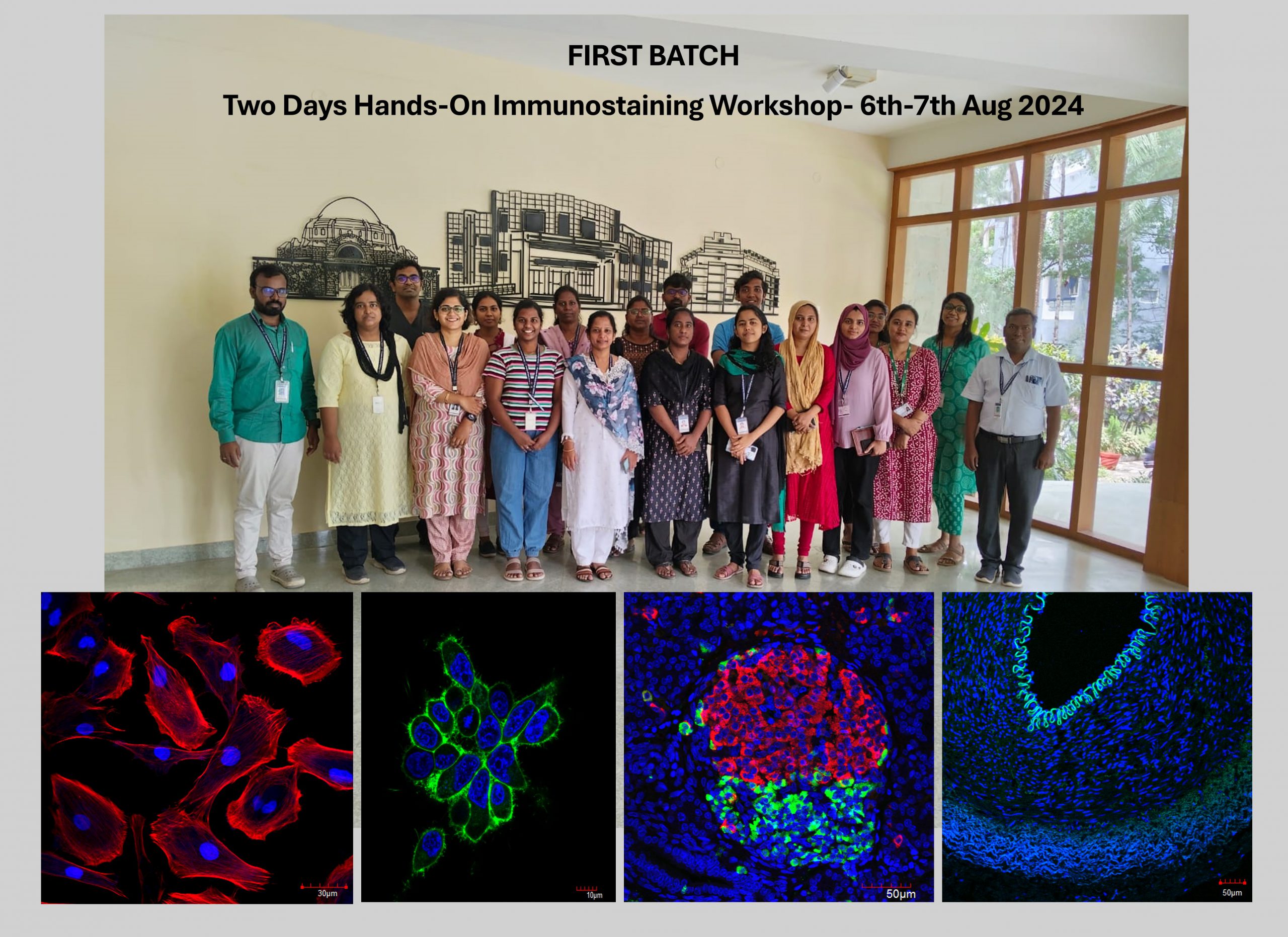
Immunostaining Hands-on workshop
The first successful Two-Day Hands-On Immunostaining Workshop was conducted on August 6th-7th, 2024, providing on-site training in routine, special, and immunostaining techniques, as well as the use of bright field, immunofluorescence, and confocal microscopes.
Radio Isotopes Lab
Description
We recently obtained the approval (AERB/RSD/Res-Plan/TN-01/2009)from Atomic Energy Regulatory Board to use radioactive materials in our center, we have thus established a dedicated facility to handle alpha and beta radioisotopes. This facility is equipped with following detection and quantification equipment.
Liquid scintillation counter (Perkin Elmer - TriCarb 2810TR) to detect the concentration and the half-life of the radioisotopes. Phosphor Imager (GE - Storm 865) to read the radiation exposed screens. GM-counter (Ludlum) a handheld detector to detect the spill.
Dr. Sandya Rani B
Fellow-E (Scientist-D)
Mr. Rajesh A
Technical Officer
Mr. Joseph Joel N
Technician
Mr. Abdul Muthallib
Technician
Dr. Shaji R.V
Faculty Support
Dr. Sanjay Kumar
Faculty Support
Email : cscrcore@cmcvellore.ac.in
Phone : 0416-228-5122/5123/5131